Well, back to the facts. Anyone patting themselves on the back hasn't read the bill (it will be back - just wait for the next food contamination scare). Every `blog' I've read since this bill hit the web has falsely listed rules for ALREADY REGULATED FACILITIES (have been since 2002) as somehow being applied to EVERYONE. Supplements defined as foods!, oh no! (have been so defined since 1994). The bill specifically excluded farms (didn't have to as the bill conformed with 415 of the FDCA as amended in 2002).
The Tester Amendment was a sop to all those who didn't read the bill (same exclusions as existed in 2002) and, probably hacked off some small producer who was not yet regulated but who, because he/she/it made over $500,000 would now be regulated (purely arbitrary dollar amount to appease `vox populi').
This bill was opposed, it seems, by those small/medium sized producers that were ALREADY REGULATED. They are required to be inspected (not enough inspectors), they are required to maintain specific records (though they can simply refuse to show them as the FDA had no authority to demand them - specific change for regulated facilities re: fines). The bill also provided the FDA more latitude in dealing with the food products entering the United States.
So, everyone is still, today, operating under the Food Drug and Cosmetic Act as Amended by the 2002 Food Safety and Bioterrorism Act - same exclusions/same definitions of what constitutes a regulated facility that this bill WOULD NOT have changed at all.
I'm reposting the actual language so, when this comes up again (e.coli in spinach/Salmonella in tomatoes/Exotic Newcastle Disease in Poultry/e.coli in Nutmeg?) I'll just link back to it.
From the Bill:
FDC Act (Chapter IV (U.S.C. Subchapter 4) Food Maintenance and inspection of Records (FD&C Act # 414./U.S. Code Section # - 21 U.S.C 350c.)
http://www.fda.gov/RegulatoryInform...icActFDCAct/FDCActChapterIVFood/ucm107899.htm
`Facilities Defined': Registration of Food Facilities (FD&C Act # 415/U.S. Code Section # - 21 U.S.C. 350d)
http://www.fda.gov/RegulatoryInform...icActFDCAct/FDCActChapterIVFood/ucm107910.htm
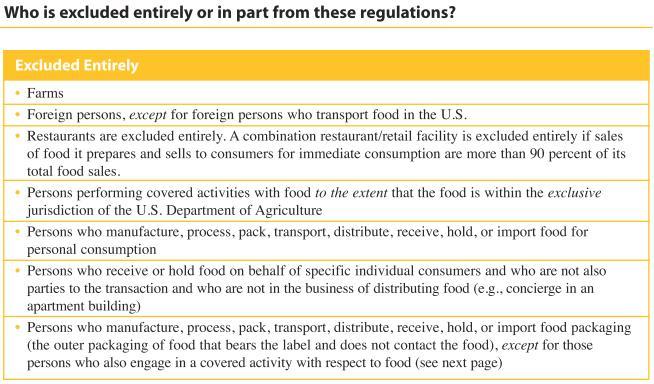
List of exclusions - those who won't be bothered: (FDA's implementation of the 2002 Bioterrorism Act)
http://www.fda.gov/downloads/Food/G...FoodDefenseandEmergencyResponse/UCM113920.pdf
Exclusions from the pamphlet linked to above:
Big producers could care less about backyarders - they comply with all regs as they don't want to lose market share. If we were in business we'd want excellent inspection of both ourselves and our competitors as some fool down the road with 300 chooks who knows that production birds will produce like clock work even if poorly cared for, who buys OTC antibiotics and keeps the birds dosed because he can't be bothered with cleaning them up, and then sells contaminated eggs alongside ours -- then all producers are suspect. When this happens on a large scale (like the Exotic Newcastle outbreak in California) other countries simply place a ban on the foodstuff and those who play by the rules are screwed.
The Inspector General of the Department of Health and Human Services examined the FDA's inspections back in 4/10 (why the FDA was given authority through civil action - in the language of the bill).
Increase the frequency of food facility inspections, with particular emphasis on high-risk facilities.
Provide additional guidance about when it is appropriate to lower OAI classifications.
Take appropriate actions against facilities with OAI classifications, particularly those that have histories of violations.
Ensure that violations are corrected for all facilities that receive OAI classifications.
Consider seeking statutory authority to impose civil penalties through administrative proceedings against facilities that do not voluntarily comply with statutory and regulatory requirements.
Seek statutory authority to allow FDA access to facilities records during the inspection process.
http://www.oig.hhs.gov/oei/reports/oei-02-08-00080.pdf
If one is going to scream about the awful government, it is best go to the source and see if it is worth one's time, instead of relying on talking heads and folks who have their own reasons to whip the froth.