Quote: Yep, that happens.
Even though I had some experience using microscopes, with those fecal slides I spent hours going cross-eyed trying to figure out what I was seeing.
1) Make sure you've got your optics setup for your eyes (e.g., webs.wofford.edu/davisgr/histology/using_a_microscope.doc)
2) Make sure you don't have to bend to look into the microscope - good posture saves your neck! It's really bad when your arms go numb!
3) Take breaks - even short ones. Look at the far end of the room to relax your eye muscles.
I'm using the microscope camera software in full screen mode and watching my computer monitor instead of looking through the microscope eye pieces. Much better!!! I have so many "floaters" in my eyes that I mostly see those, not the stuff on the slide. It's scary even though I know those are protein clumps not microbes/monsters.
After you have the optics aligned for your eyes, take a new slide. Make an "X" on the slide. Make a "+" on a cover slip. Add a drop of water to the slide, then add the cover slip, + side down. You can even use different colors of ink for the 2 marks. Use the lowest magnification to focus on the +, then use the gross focus to find the lower (X) mark. In observing slides, you will be focusing up and down between these 2 depths to be sure to catch all the critters.
When you're trying to bring something into focus, use the position knobs to rock the stage back & forth (or forward & back) while you dial in the focus, then you'll know you are focusing on the slide contents and not some crud elsewhere in the light path.
After you focus on something using the lowest magnification, you should be able to see it as a blob at the next higher magnification. Place the object of interest in the center of the field of view to be sure to find it again. Use the fine focus to bring it into clear view. Repeat for the next higher magnification.
When you go up to the highest magnification (the lens will say something like 100x OIL), you need to put a drop of microscope optical oil on the slide, and one on the condenser lens - the condenser is the stuff below the stage that focuses the light beam - then raise the condenser until the oil touches the bottom of the slide. Open the condenser iris to get enough light. No worries, the oil doesn't mess up the view when you need to go to a lower power again. When you are done, be sure to wipe off the oil with lens paper or you'll have a dust ball. You can use some alcohol, too, if needed.
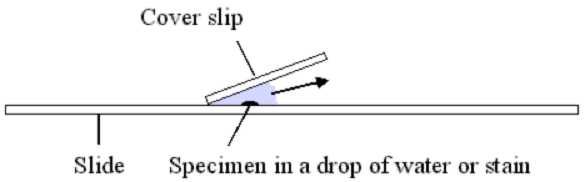
A tip about cover slips - you can reduce the bubbles caught underneath by touching one edge of the cover slip to the liquid, rotate the top of the cover slip towards the slide - the liquid will wick up the cover slip. Once it's ~1/4 way up, let go of the cover slip so it drops onto the liquid. Tap the cover slip a couple times to force out any bubbles. If that doesn't work, drop more liquid on the slide to the left of the cover slip and use absorbent paper touched to the right side of the cover slip to draw fluid through and hopefully carry any bubbles away. I use saline (for contacts
 ).
).

There are dozens of helpful microscope how-to website and videos online if the above isn't clear. I like this one:
http://bitesizebio.com/13393/what-everybody-ought-to-know-about-the-light-microscope/
Good luck!
Even though I had some experience using microscopes, with those fecal slides I spent hours going cross-eyed trying to figure out what I was seeing.
1) Make sure you've got your optics setup for your eyes (e.g., webs.wofford.edu/davisgr/histology/using_a_microscope.doc)
2) Make sure you don't have to bend to look into the microscope - good posture saves your neck! It's really bad when your arms go numb!
3) Take breaks - even short ones. Look at the far end of the room to relax your eye muscles.
I'm using the microscope camera software in full screen mode and watching my computer monitor instead of looking through the microscope eye pieces. Much better!!! I have so many "floaters" in my eyes that I mostly see those, not the stuff on the slide. It's scary even though I know those are protein clumps not microbes/monsters.
After you have the optics aligned for your eyes, take a new slide. Make an "X" on the slide. Make a "+" on a cover slip. Add a drop of water to the slide, then add the cover slip, + side down. You can even use different colors of ink for the 2 marks. Use the lowest magnification to focus on the +, then use the gross focus to find the lower (X) mark. In observing slides, you will be focusing up and down between these 2 depths to be sure to catch all the critters.
When you're trying to bring something into focus, use the position knobs to rock the stage back & forth (or forward & back) while you dial in the focus, then you'll know you are focusing on the slide contents and not some crud elsewhere in the light path.
After you focus on something using the lowest magnification, you should be able to see it as a blob at the next higher magnification. Place the object of interest in the center of the field of view to be sure to find it again. Use the fine focus to bring it into clear view. Repeat for the next higher magnification.
When you go up to the highest magnification (the lens will say something like 100x OIL), you need to put a drop of microscope optical oil on the slide, and one on the condenser lens - the condenser is the stuff below the stage that focuses the light beam - then raise the condenser until the oil touches the bottom of the slide. Open the condenser iris to get enough light. No worries, the oil doesn't mess up the view when you need to go to a lower power again. When you are done, be sure to wipe off the oil with lens paper or you'll have a dust ball. You can use some alcohol, too, if needed.
A tip about cover slips - you can reduce the bubbles caught underneath by touching one edge of the cover slip to the liquid, rotate the top of the cover slip towards the slide - the liquid will wick up the cover slip. Once it's ~1/4 way up, let go of the cover slip so it drops onto the liquid. Tap the cover slip a couple times to force out any bubbles. If that doesn't work, drop more liquid on the slide to the left of the cover slip and use absorbent paper touched to the right side of the cover slip to draw fluid through and hopefully carry any bubbles away. I use saline (for contacts

There are dozens of helpful microscope how-to website and videos online if the above isn't clear. I like this one:
http://bitesizebio.com/13393/what-everybody-ought-to-know-about-the-light-microscope/
Good luck!




