Lochte Farms
In the Brooder
So, I have two Ameracaunas, two EE's, a Black Copper Maran, two silkie hens, two polish chickens (one is frizzled), 3 Barred Rocks, a Cochin and a Croad Langshan. Obviously the light brown, cream, and white eggers I'm not concerned about, but I would like my EE's and Ameracaunas to lay nice vibrant blue/green(ee) eggs. I'd really like for my Maran to lay a little bit darker as well, her egg is lighter than I had expected it to be, but I didn't purchase her from a big time breeder or anything so I know she isn't going to lay dark chocolate eggs. I'm still waiting to see what my Langshan produces (cross your fingers for a beautiful plum egg! but I'm yee of little faith). I spoke to a guy in one of our local forums that shows chickens and eggs, but he was reluctant to tell me what he feeds his birds for beautiful vibrant eggs. All he would tell me was that if I wanted a BLUE egg I should feed the chicken something BLUE. Okay... So I'm thinking blueberries or blackberries. I don't want to give them anything harmful, they're my babies and I'd fall to pieces if they got sick because of something I did to them. So I'm here to ask y'all  Has anyone experimented with different types of treats that have impacted the color of their eggs (the shells, not the yolks, we already have super vibrant, almost neon orange yolks)? My girls are free range and they all get treats together, so I figure if the blackberries do impact their shells, my green layer might produce more aqua or turquoise colored eggs. I'm good with that! This is what I get as of now
Has anyone experimented with different types of treats that have impacted the color of their eggs (the shells, not the yolks, we already have super vibrant, almost neon orange yolks)? My girls are free range and they all get treats together, so I figure if the blackberries do impact their shells, my green layer might produce more aqua or turquoise colored eggs. I'm good with that! This is what I get as of now  I really can't complain, to be honest, it was just a curiosity now that the thought has been put in my head lol
I really can't complain, to be honest, it was just a curiosity now that the thought has been put in my head lol


 Has anyone experimented with different types of treats that have impacted the color of their eggs (the shells, not the yolks, we already have super vibrant, almost neon orange yolks)? My girls are free range and they all get treats together, so I figure if the blackberries do impact their shells, my green layer might produce more aqua or turquoise colored eggs. I'm good with that! This is what I get as of now
Has anyone experimented with different types of treats that have impacted the color of their eggs (the shells, not the yolks, we already have super vibrant, almost neon orange yolks)? My girls are free range and they all get treats together, so I figure if the blackberries do impact their shells, my green layer might produce more aqua or turquoise colored eggs. I'm good with that! This is what I get as of now  I really can't complain, to be honest, it was just a curiosity now that the thought has been put in my head lol
I really can't complain, to be honest, it was just a curiosity now that the thought has been put in my head lol

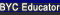



 And thank you Penny/LittleRedFace! This is what I picked up from the nesting boxes yesterday. We get around 6 eggs a day with 7 layers, my bowl full of eggs was a collection of a few days for a "photoshoot"
And thank you Penny/LittleRedFace! This is what I picked up from the nesting boxes yesterday. We get around 6 eggs a day with 7 layers, my bowl full of eggs was a collection of a few days for a "photoshoot"  Hopefully chicken math doesn't kick in bc we've got a line of people waiting for eggs as it is.
Hopefully chicken math doesn't kick in bc we've got a line of people waiting for eggs as it is. 